Scientists from the University of Oulu in Finland, in collaboration with their counterparts at the University of Tartu in Estonia, have identified the genetic loci associated with pneumonia susceptibility and confirmed the causal relationship between lifestyle factors such as smoking and obesity and pneumonia onset through large-scale genomic research. The findings, published online in early February 2026 in EBioMedicine, a leading international open-access journal focused on translational medicine and precision health research, provide crucial scientific evidence for understanding the genetic basis of pneumonia and formulating personalized prevention strategies.
Pneumonia is a common yet potentially fatal lower respiratory tract infection that leads to a large number of hospitalizations worldwide each year, posing a severe threat especially to the elderly, patients with chronic lung diseases and individuals with weakened immune function. While environmental and behavioral factors like smoking, excessive alcohol consumption and malnutrition have long been recognized as major risk factors for pneumonia, the underlying biological mechanisms – particularly the interaction between genetic susceptibility and environmental factors – have lacked supporting evidence from large population studies.
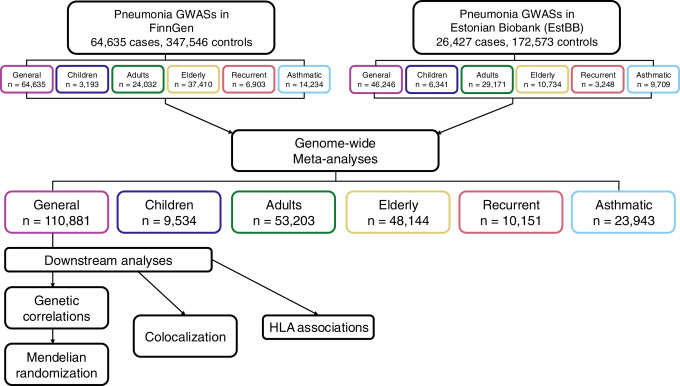
For this research, the team integrated genome-wide association study (GWAS) data from more than 600,000 participants across Finland and Estonia with decades of electronic health records, covering multi-dimensional information including hospital diagnoses, medication use and lifestyle questionnaires. Using advanced statistical genetics methods, the researchers identified 12 genomic loci significantly linked to pneumonia risk, 8 of which were discovered for the first time globally. These newly found genetic regions are mostly involved in the innate immune response, inflammatory regulatory pathways and pulmonary epithelial barrier function, such as the FCGR2A gene near chromosome 11q23.3. This gene is known to encode Fcγ receptor (FcγR) that play a key role in antibody-mediated pathogen clearance.
Notably, the research team adopted Mendelian Randomization (MR), a causal inference tool, and used genetic variants as instrumental variables to eliminate the interference of confounding factors. The results clearly demonstrated that smoking – measured by daily cigarette consumption and nicotine dependence, and obesity – assessed by body mass index (BMI) both significantly increase the risk of pneumonia. Specifically, each one-standard-deviation increase in genetically predicted BMI (approximately 4.5 kg/m²) was associated with an 18% rise in pneumonia risk. Genetically predicted smoking initiation and cigarette consumption were both positively correlated with the risk of hospital-admitted pneumonia by about 20-30% (odds ratio OR≈1.22-1.3).
The study also revealed heterogeneous characteristics across different populations. In groups of patients with recurrent pneumonia or those aged over 75 years, genes related to nicotine metabolism and addiction, such as the CHRNA5-A3-B4 gene cluster, showed stronger association signals. This suggests that smoking may cause more persistent damage to the pulmonary defense system of high-risk populations.
Markku Peltonen, Professor at the Department of Public Health and Clinical Nutrition of the University of Oulu, stated: “This study not only expands our understanding of the genetic architecture of pneumonia, but more importantly, it provides genetic evidence to support the public health claim that modifying lifestyle habits can reduce pneumonia risk. Smoking cessation and weight management should not only be regarded as measures for chronic disease prevention and control, but also integrated into the primary prevention system for respiratory infections.”
Andres Metspalu, Director of the Institute of Genomics at the University of Tartu, added: “The comprehensive national health registration systems in Nordic countries offer a unique advantage for such large-scale biomedical research. In the future, our team plans to expand the data to other European populations and explore the potential impacts of gene-environment interactions on vaccine response and antibiotic efficacy.”
Experts point out that with the development of multi-omics technologies, risk stratification based on individual genetic background is expected to guide the early screening of high-risk populations, the prioritization of vaccination and even the formulation of personalized interventions in the future.
According to published data from the World Health Organization (WHO), pneumonia remains one of the leading causes of death among children and the elderly worldwide. This study emphasizes that in addition to promoting vaccination and improving healthcare access, efforts should be made to reduce modifiable risk factors at the source, with a particular focus on tobacco control and obesity prevention and management. These interventions are of far-reaching significance for alleviating the disease burden of pneumonia caused by pneumonia.
